FEBS Open Bio (2025) 15: 383-398 https://doi.org/10.1002/2211-5463.13944
- tuberculosis-lbp
- 4 déc. 2024
- 1 min de lecture
Dernière mise à jour : 20 juin 2025
Antitubercular potential and pH-driven mode of action of salicylic acid derivatives
Laudouze, J.; Francis, T.; Forest, E.; Mies, F.; Bolla, J.M.; Crauste, C.; Canaan, S.; Shlyonsky, V.; Santucci, P.; Cavalier, J.-F.
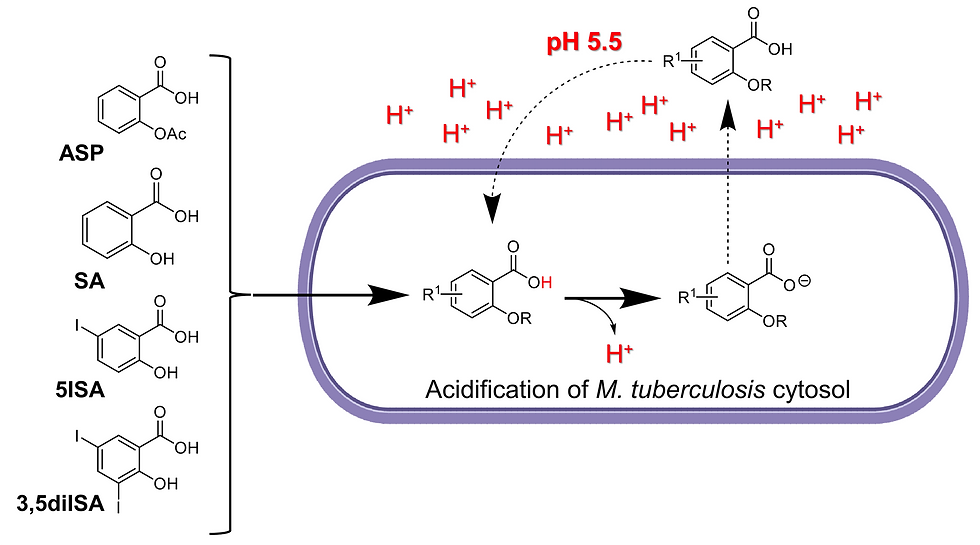
In the search for new antituberculosis drugs with novel mechanisms of action, we evaluated the antimycobacterial activity of a panel of eight phenolic acids against four pathogenic mycobacterial model species, including Mycobacterium tuberculosis. We demonstrated that salicylic acid (SA), as well as the iodinated derivatives 5-iodo-salicylic acid (5ISA) and 3,5-diiodo-salicylic acid (3,5diISA), displayed promising antitubercular activities. Remarkably, using a genetically encoded mycobacterial intrabacterial pH reporter, we describe for the first time that SA, 5ISA, 3,5diISA, and the anti-inflammatory drug aspirin (ASP) act by disrupting the intrabacterial pH homeostasis of M. tuberculosis in a dose-dependent manner under in vitro conditions mimicking the endolysosomal pH of macrophages. In contrast, the structurally related second-line anti-TB drug 4-aminosalicylic acid (PAS) had no pH-dependent activity and was strongly antagonized by l-methionine supplementation, thereby suggesting distinct modes of action. Finally, we propose that SA, ASP, and its two iodinated derivatives could restrict M. tuberculosis growth in a pH-dependent manner by acidifying the cytosol of the bacilli, therefore making such compounds very attractive for further development of antibacterial agents.